Abstract
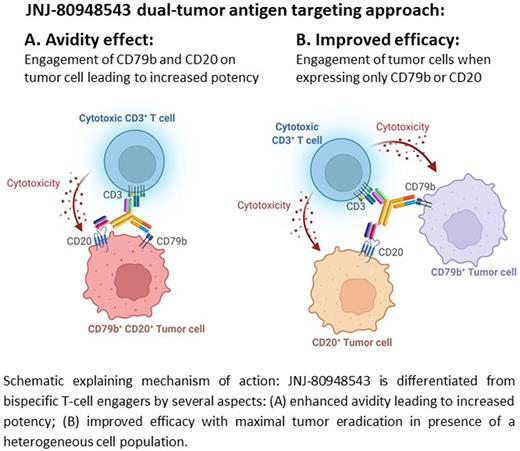
Background: B-cell non-Hodgkin lymphoma (B-NHL) is the most common hematological malignancy worldwide. Despite improvements in available therapies, relapsed/refractory (R/R) B-NHLs are characterized by poor prognosis. Immunotherapies engaging T cells, such as chimeric antigen receptor T cells (CAR-T) or bispecific cluster of differentiation (CD)3 redirection antibodies, show promising response rates in the clinic. T-cell redirection using antibodies that recognize CD3 on T cells and specific antigens on malignant cells is a novel treatment modality that may help patients whose disease no longer responds to prior lines of therapy. To further increase the efficacy of CD3 redirection therapies in B-NHL, we designed a trispecific antibody targeting two tumor antigens. CD79b and CD20 are well-validated therapeutic targets with their expression restricted to the B cell lineage and expressed in most B-cell malignancies, making them ideal T-cell redirection targets. Dual antigen recognition on B-NHL cells with a trispecific T-cell redirecting antibody has the potential to enhance tumor binding through avidity effects, maximize tumor eradication in the presence of a heterogeneous cell population, and prevent resistance via tumor antigen escape.
Methods: JNJ-80948543 is a fully human immunoglobulin G1 trispecific antibody composed of an anti-CD3ɛ single-chain variable fragment (scFv), an anti-CD20 scFv, and an anti-CD79b fragment antigen-binding (Fab) domain and an effector-silent Fc. It was selected based on biophysical assessment, low-affinity CD3 engagement, and observed therapeutic efficacy in multiple lymphoma models.
Results: JNJ-80948543 demonstrated high affinity and stable binding over 48 hours in lymphoma cells expressing varying endogenous CD79b and CD20 receptor densities. In contrast, low-affinity binding to primary human T cells expressing CD3 was observed. In vitro, JNJ-80948543 cytotoxicity was observed in a broad panel of lymphoma cells and in primary B cells. T-cell activation was observed with low cytokine secretion. Moreover, JNJ-80948543 mediated cytotoxicity of single target expressing tumor cells (CD79b or CD20) but demonstrated >1,000-fold greater potency towards cells expressing both CD79b and CD20, consistent with an avidity effect. Importantly, there was no impact on target cell viability or T cell activation in co-culture with antigen-negative cells. In vivo, JNJ-80948543 prevented the growth of CARNAVAL (GCB-DLBCL) xenografts and induced dose-dependent regression of OCI-Ly10 (ABC-DLBCL) xenograft tumors.
Conclusions: Taken together, JNJ-80948543 is a potential first-in-class trispecific antibody for the treatment of B-NHL malignancies with high unmet medical needs. A Phase I dose-escalation study of JNJ-80948543 in patients with relapsed/refractory Non-Hodgkin's Lymphoma and Chronic Lymphocytic Leukemia (CLL) will be initiated.
Disclosures
Kuchnio:Janssen: Current Employment. Yang:Janssen: Ended employment in the past 24 months. Vloemans:Janssen: Current Employment. Lowenstein:Janssen: Ended employment in the past 24 months. Cornelissen:Janssen: Current Employment. Amorim:Janssen: Current Employment. Han:Janssen: Ended employment in the past 24 months. Sukumaran:Janssen: Current Employment. Janssen:Janssen: Current Employment. Suls:Janssen: Current Employment. Bekkers:janssen: Current Employment. Lasorsa:Janssen: Current Employment. Fontana:Janssen: Current Employment. Medeiros:Janssen: Current Employment. Wu:Janssen: Current Employment. Chen:Janssen: Current Employment. Feldkamp:Janssen: Current Employment. Wu:Janssen: Current Employment. Habte:Janssen: Current Employment. Krayer:Janssen: Consultancy. Holland:Janssen: Current Employment. Deyoung:Janssen: Current Employment. Elsayed:Janssen: Current Employment. Philippar:Janssen: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal